Research
Role of peritoneal macrophages in colorectal tumor metastasis
Colorectal cancer is the third most common cancer and the fourth most common cause of cancer-associated death worldwide, being the metastatic disease the principal cause of mortality. Besides the lymphatic and hematogenous routes of metastasis, colorectal cancer gives rise to tumor cell dissemination in the peritoneal cavity, which leads to peritoneal tumor metastasis, which develop in 5-15% of colorectal cancer patients at the time of initial diagnosis, and in 20–50% of patients with recurrence.
An in-depth understanding of the mechanisms controlling the response of the peritoneal immune system to peritoneal tumor metastasis is needed to develop immunotherapeutical treatments as an alternative to the current therapeutical stategy based on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy associated to severe adverse effects.
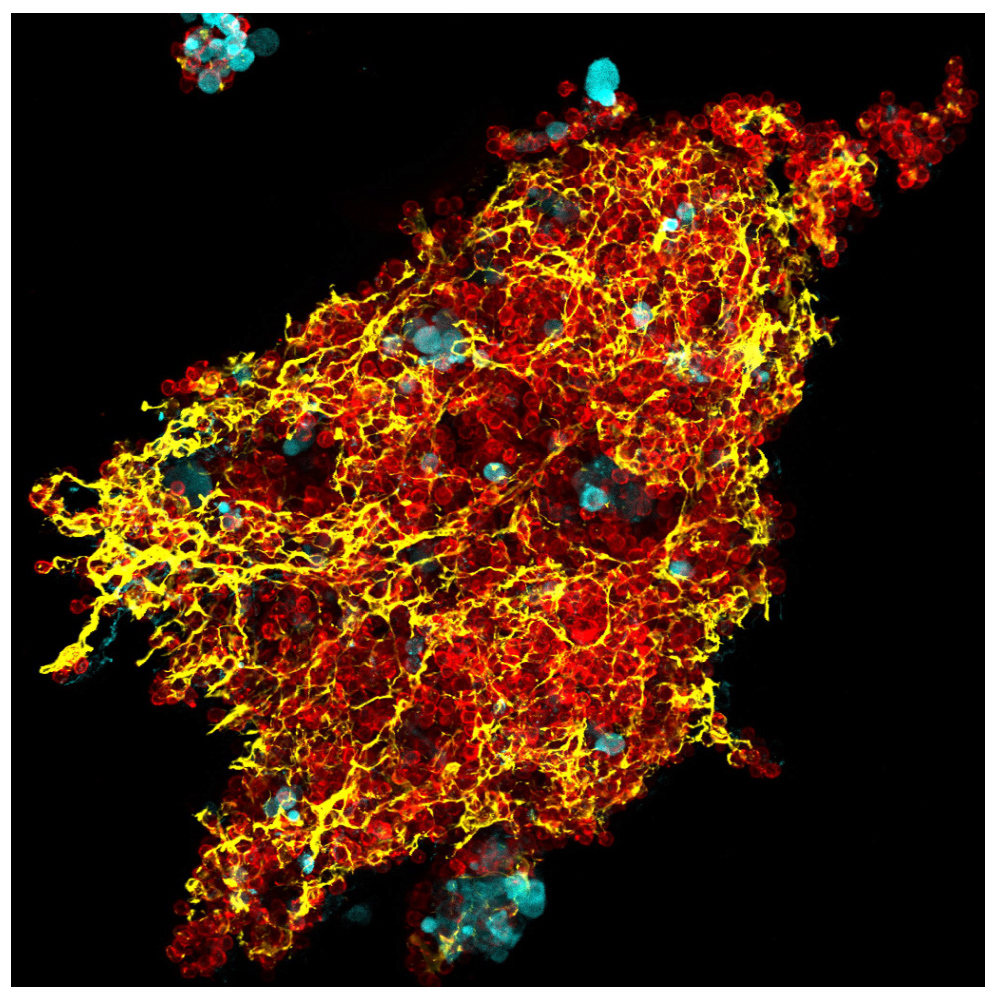
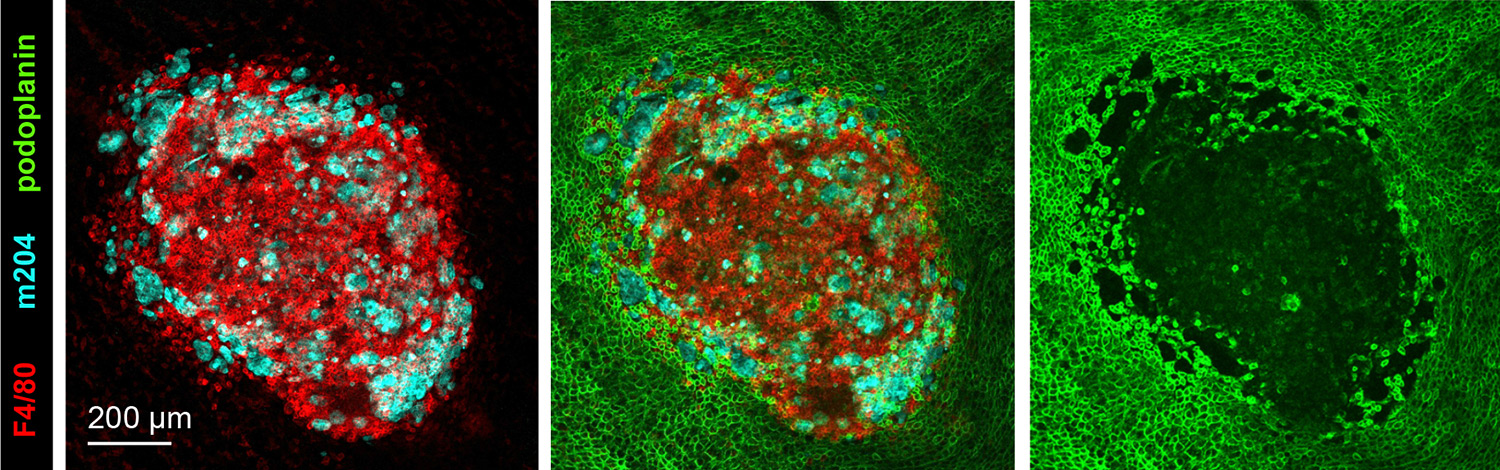
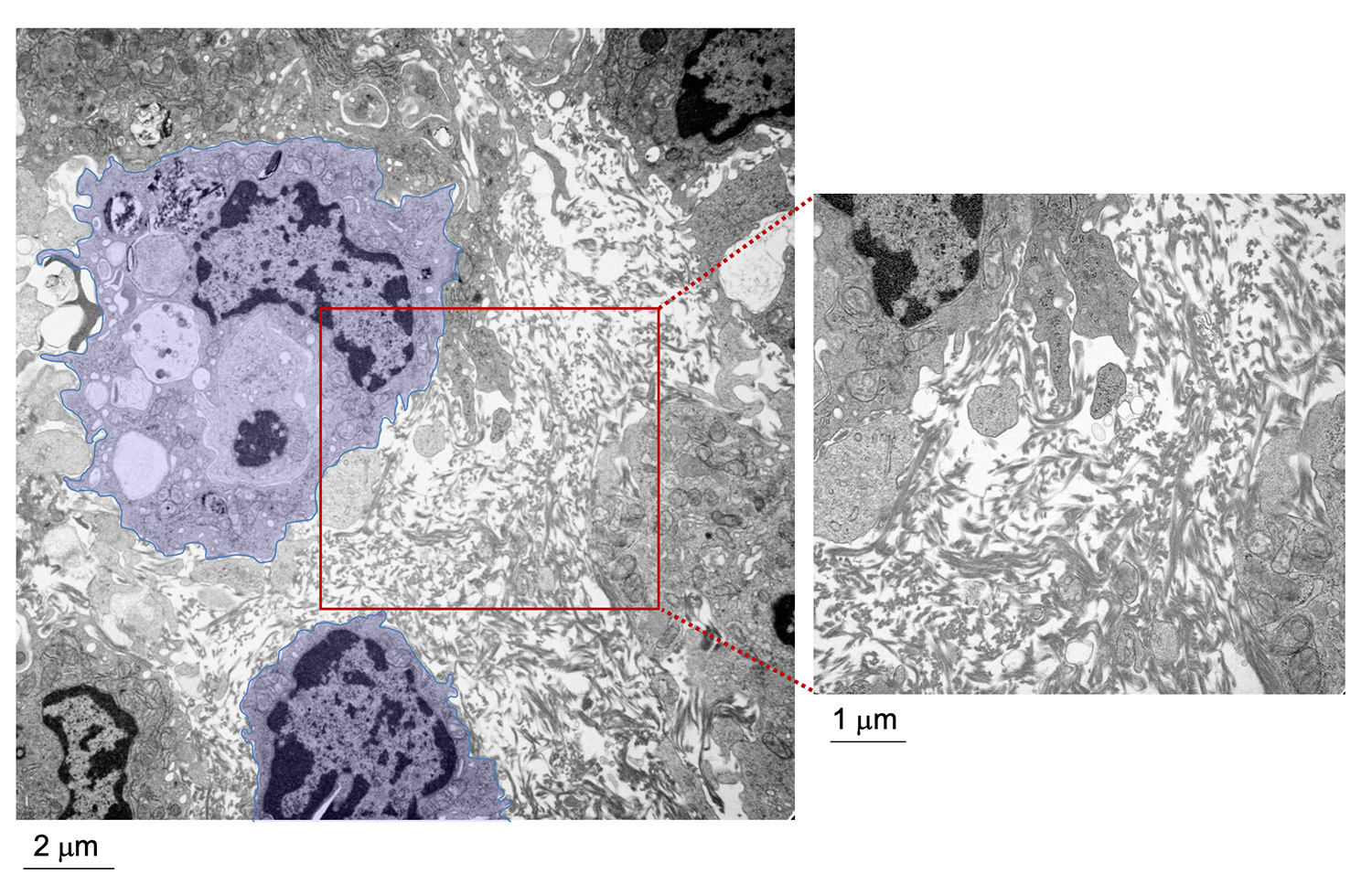
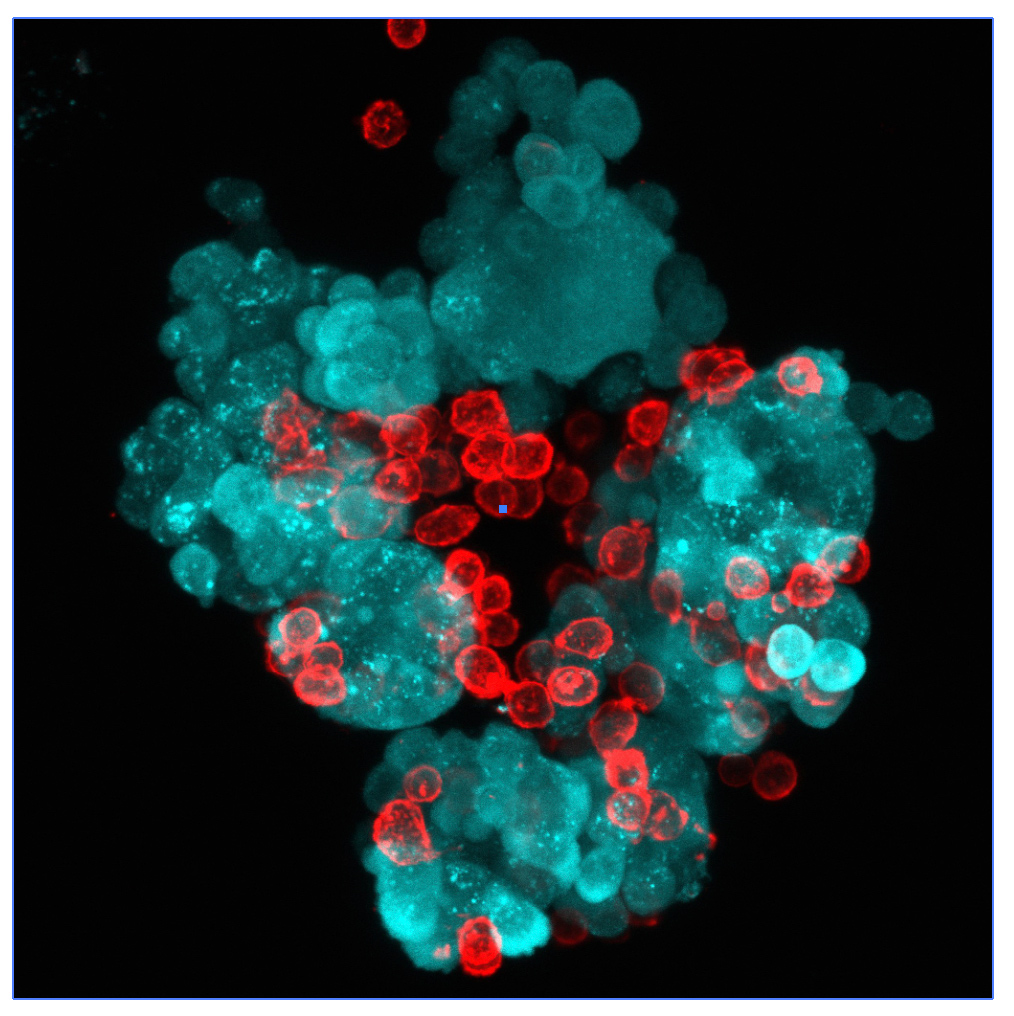
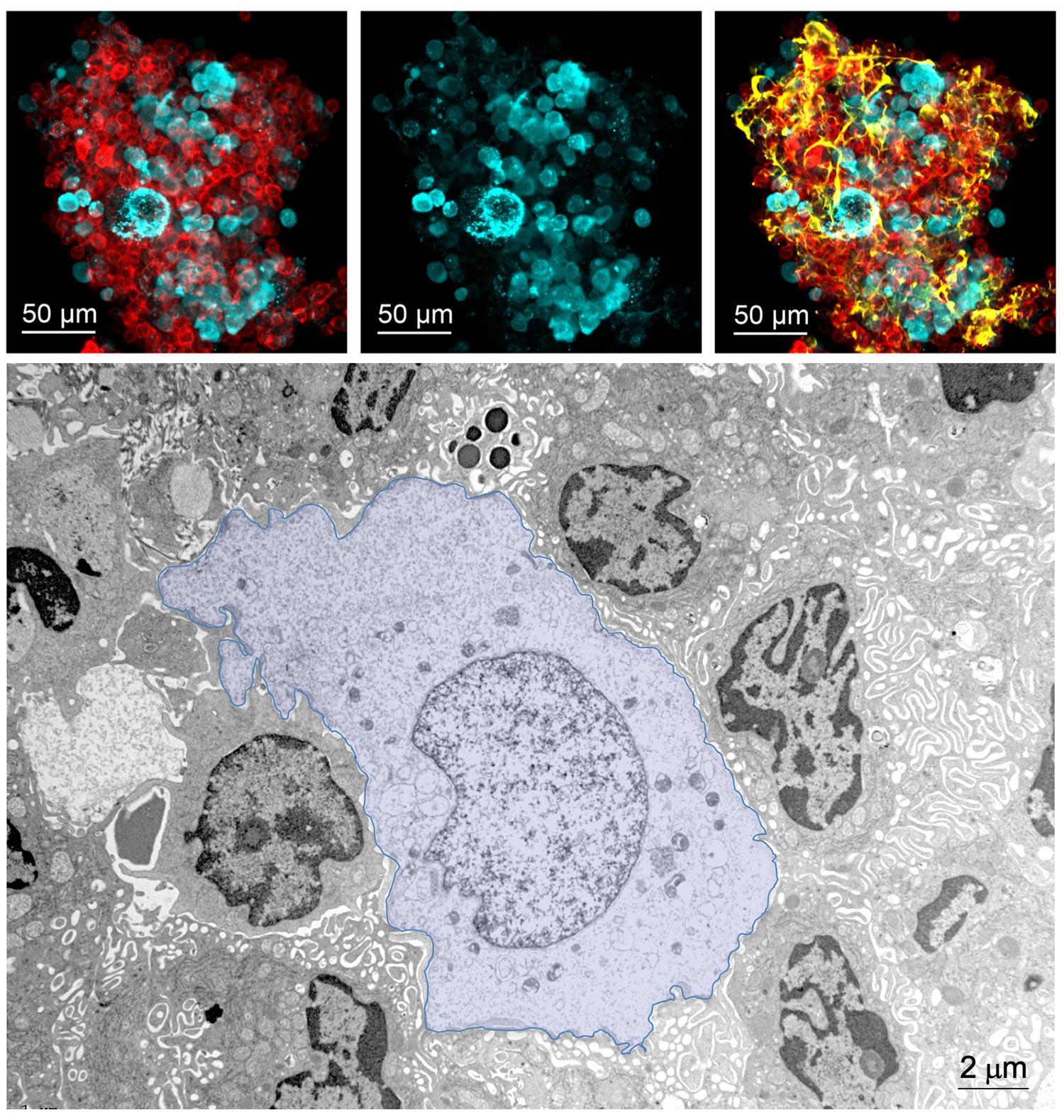
The experimental model developed in Carlos Ardavin lab to explore the response of the peritoneal innate immune system to intraperitoneal colorectal cancer metastasis relies on the intraperitoneal or intracecal transfer of mouse tumor organoids, derived from primary tumors or liver metastasis, kindly provided by Dr. Eduard Batlle (IRB, Barcelona). On going research in this area involves monitoring of metastatic growth in the peritoneal wall and omentum in control and genetically-modified mouse lines, by IVIS and whole mount immunofluorescence combined with confocal microscopy, kinetics and transcriptomic analyses of resident and recruited peritoneal cells, with the aim of exploring the interplay between peritoneal immune cells, mesothelial cells and coagulation during peritoneal metastatic growth.