Research
Innate immunity against peritoneal bacterial infection
The mechanisms linking the exacerbated inflammation caused by intra-abdominal infection to tissue damage, coagulopathy and organ dysfunction during sepsis have been extensively investigated. However, the defense mechanisms responsible for the control of intraperitoneal bacterial infection in non-lethal sepsis, remain poorly understood.
With the aim of exploring the response of peritoneal immune cells to bacterial challenge, and to understand how peritoneal immune cells, free in the peritoneal fluid in homeostasis, interact and fulfill their defense functions in the absence of an extracellular stromal support, we used an intra-abdominal sepsis model based on the intraperitoneal administration of a sublethal inoculum of E. coli, a prevalent bacterial species in sepsis caused by Gram-negative bacteria.
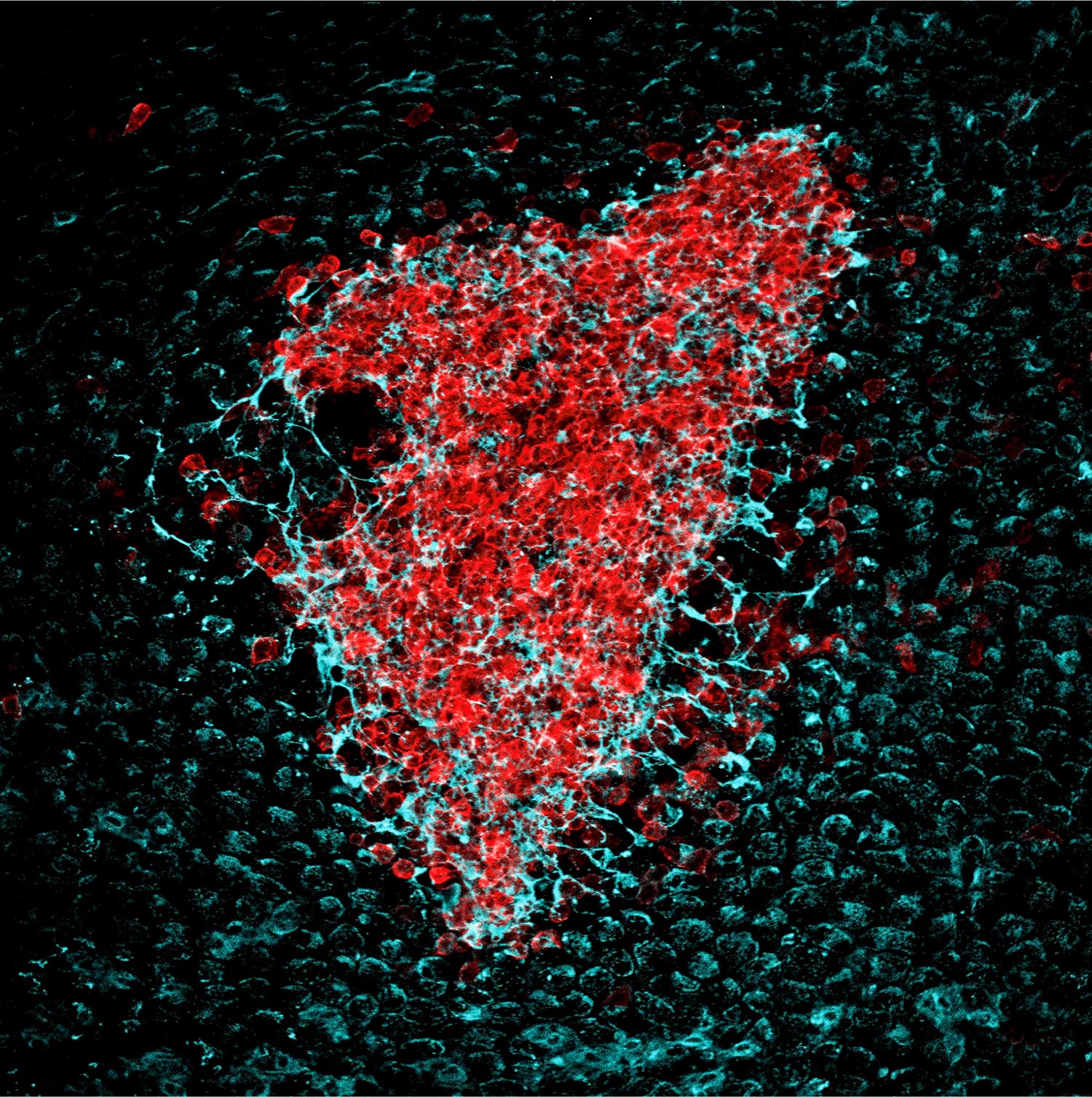
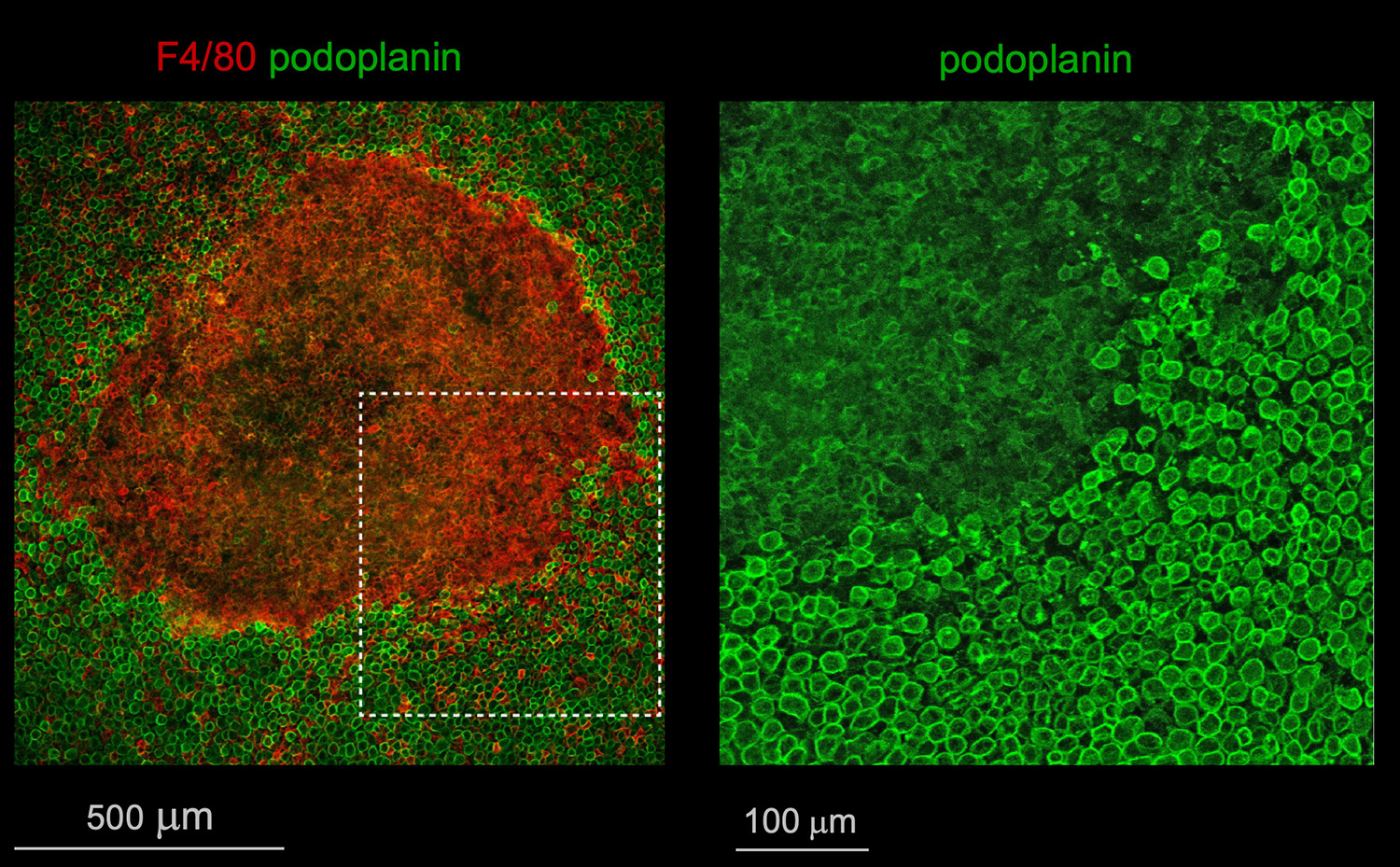
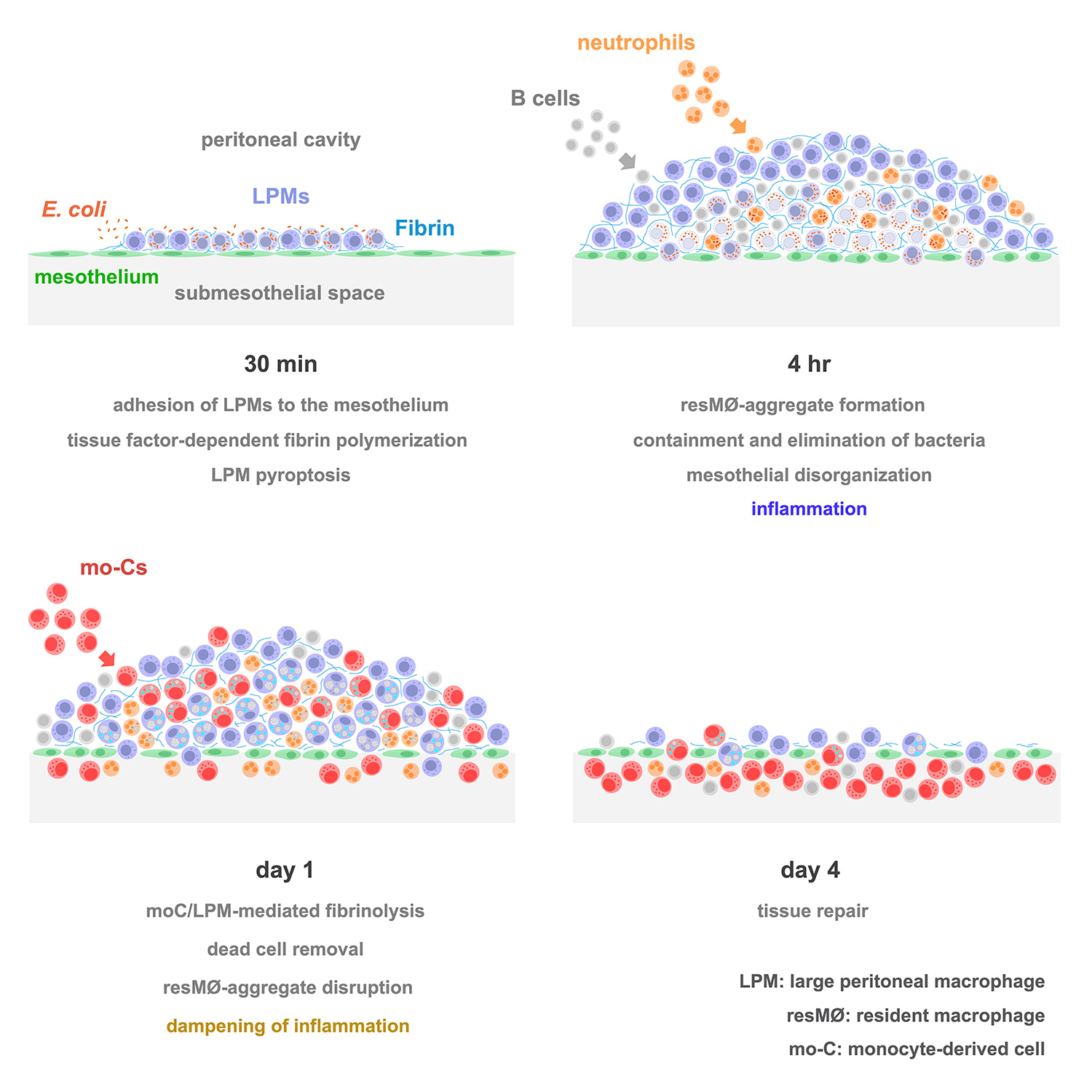
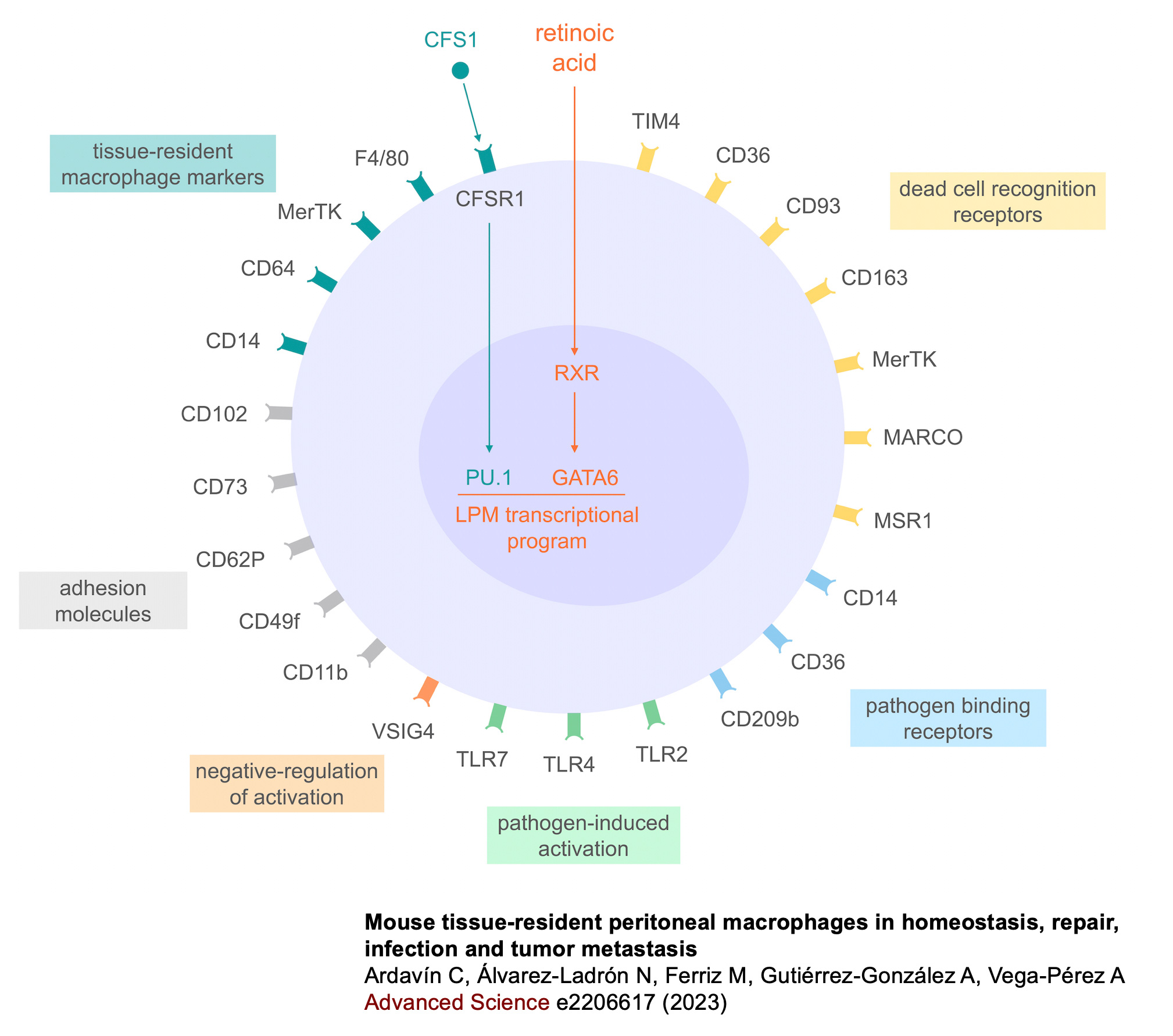
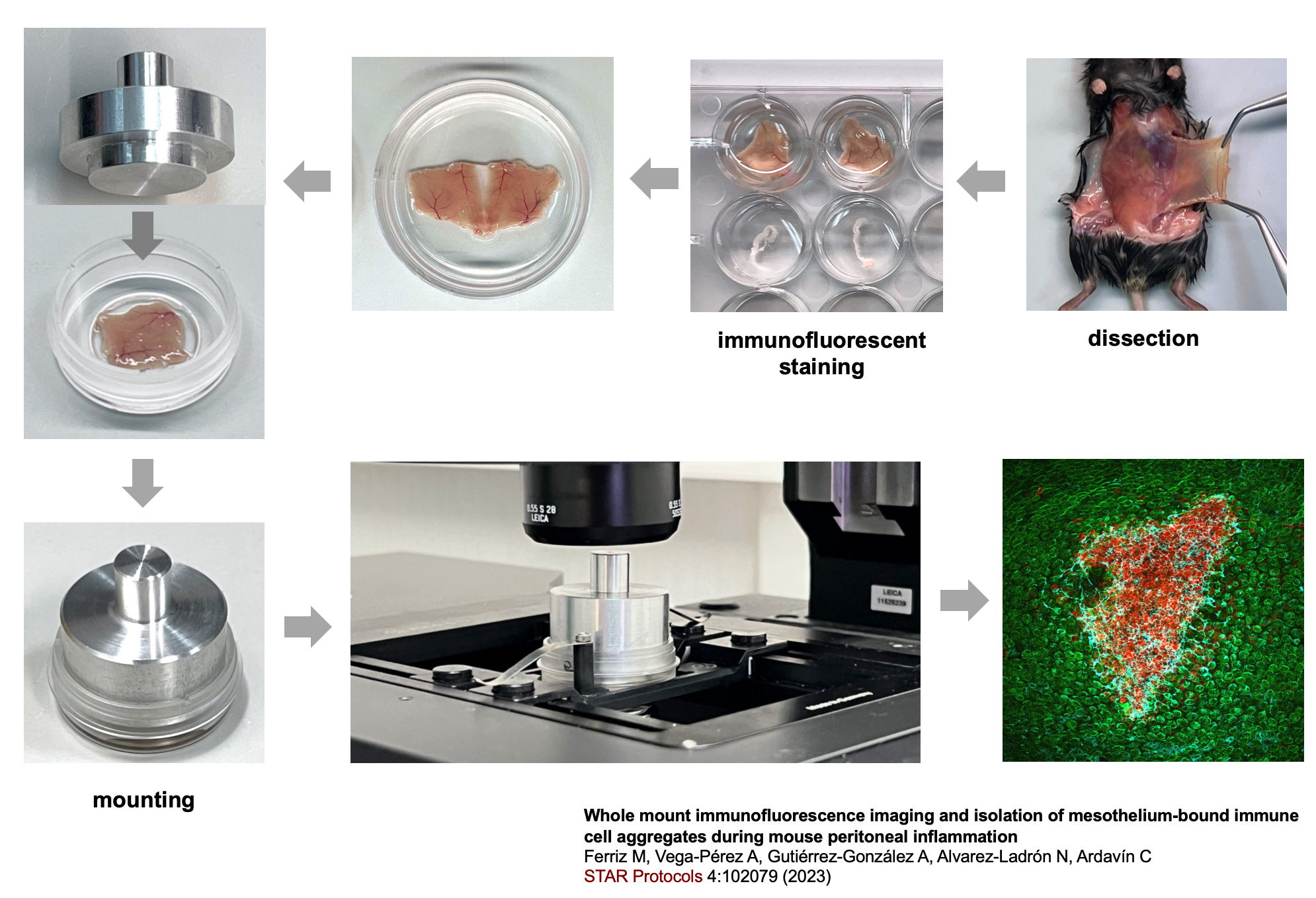
Studies carried out in Carlos Ardavín lab demonstrate that large peritoneal macrophages (LPMs), classically linked to peritoneal homeostasis, are crucial for defense against E. coli infection by coordinating the formation of mesothelium-bound, multicellular structures, composed by sequentially-recruited LPMs, B1-cells, neutrophils and monocyte-derived cells (moCs), that we termed resMØ-aggregates.
resMØ-aggregates are dynamic, transient structures, that provide a physical scaffold allowing the interaction and function of peritoneal immune cells, free in the peritoneal fluid in homeostasis, and enable efficient control of infection. The formation of resMØ-aggregates requires the development of a fibrin scafold resulting from mesothelial tissue factor-dependent fibrin polymerization, triggered by LPM-mesothelial interactions. During the resolution of infection, LPMs control the recruitment of moCs that are essential for fibrinolysis-mediated resMØ-aggregate disaggregation, and prevented peritoneal overt-inflammation (Vega-Pérez et al., Immunity 2021).
Ongoing research in Carlos Ardavin lab is focused on defining the molecular events driving fibrin polymerization during peritoneal antimicrobial immunity, and in exploring whether resMØ-aggregate-like structures may also be formed during infection in other body cavities, such as the pleural cavity or the brain ventricular system, harboring MØs in a fluidic environment, that may need to attach to the epithelium lining these cavities to fulfill their immune defense functions.
Overall these data underline an essential immune-mesothelium cooperation for an efficient peritoneal immunity and supports that defense against peritoneal bacterial infections relies on a complex interaction between inflammation, coagulation and immunity, that is largely controlled by LPMs and requires the formation of fibrin-dependent resMØ-aggregates.