Research
Closed projects
Innate immunity against systemic fungal infection
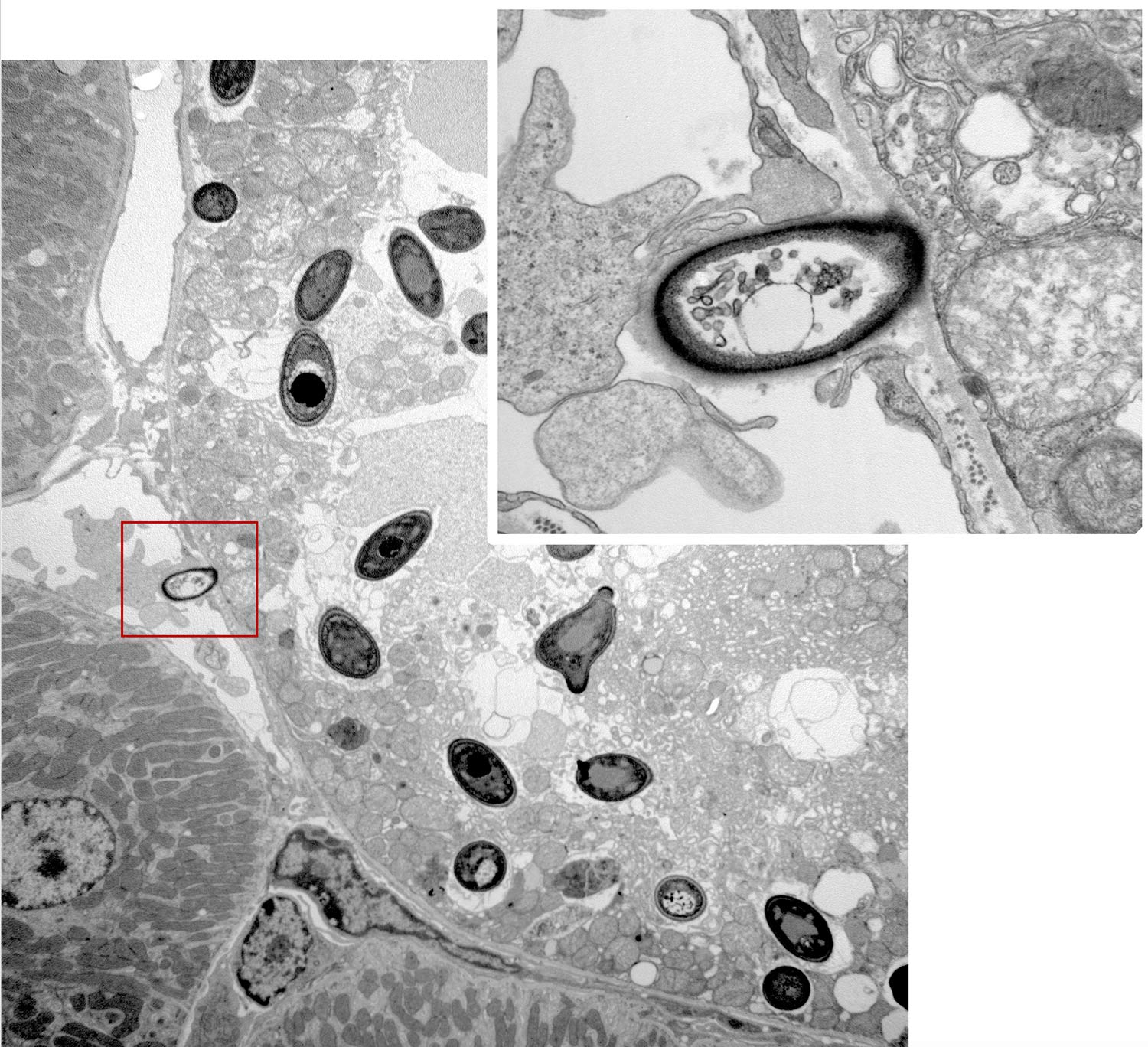
Neutrophils play a crucial role in defense against systemic candidiasis, a disease associated with a high mortality rate in patients receiving immunosuppressive therapy, although the early immune mechanisms that boost the candidacidal activity of neutrophils remain to be defined in-depth.
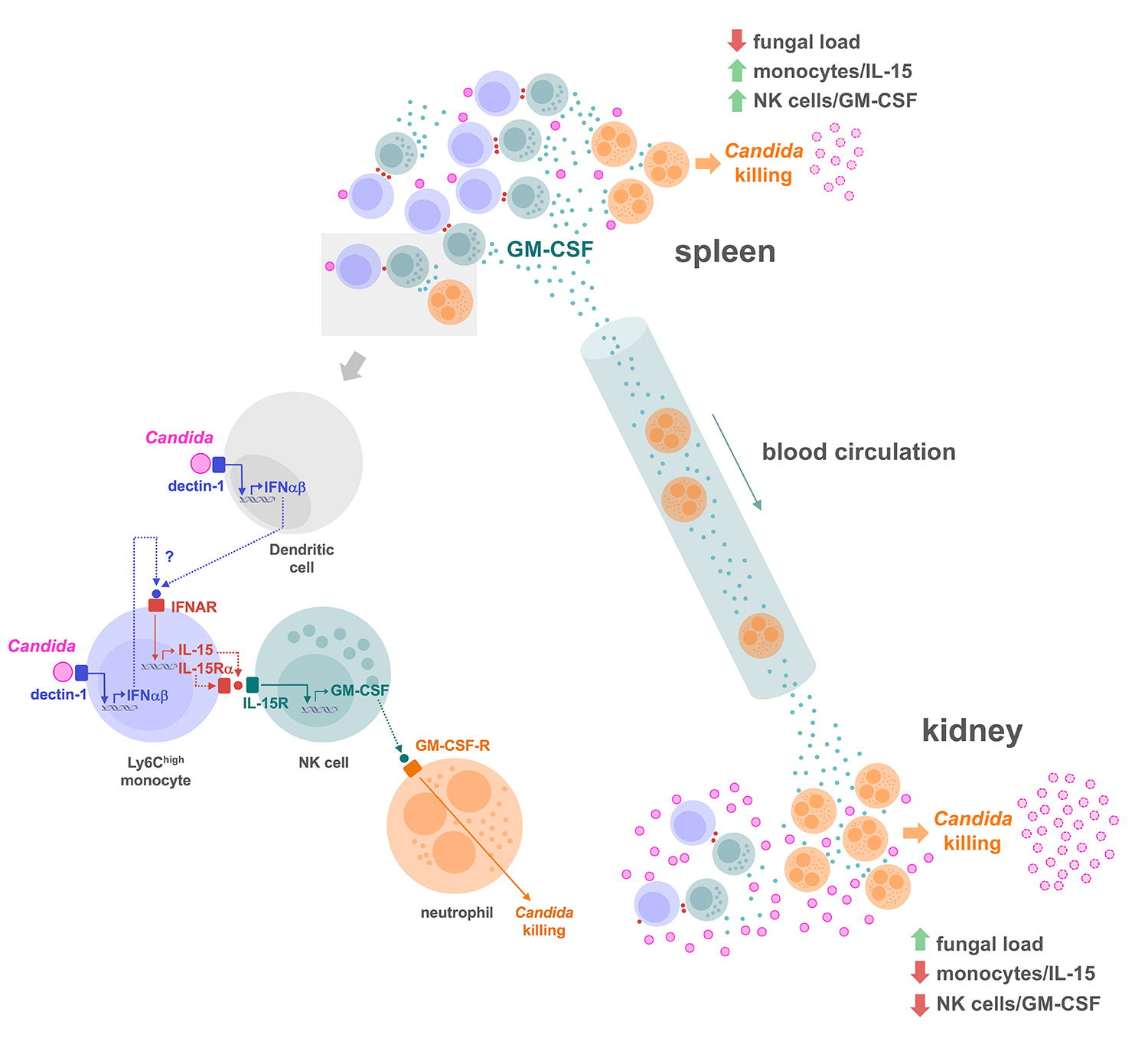
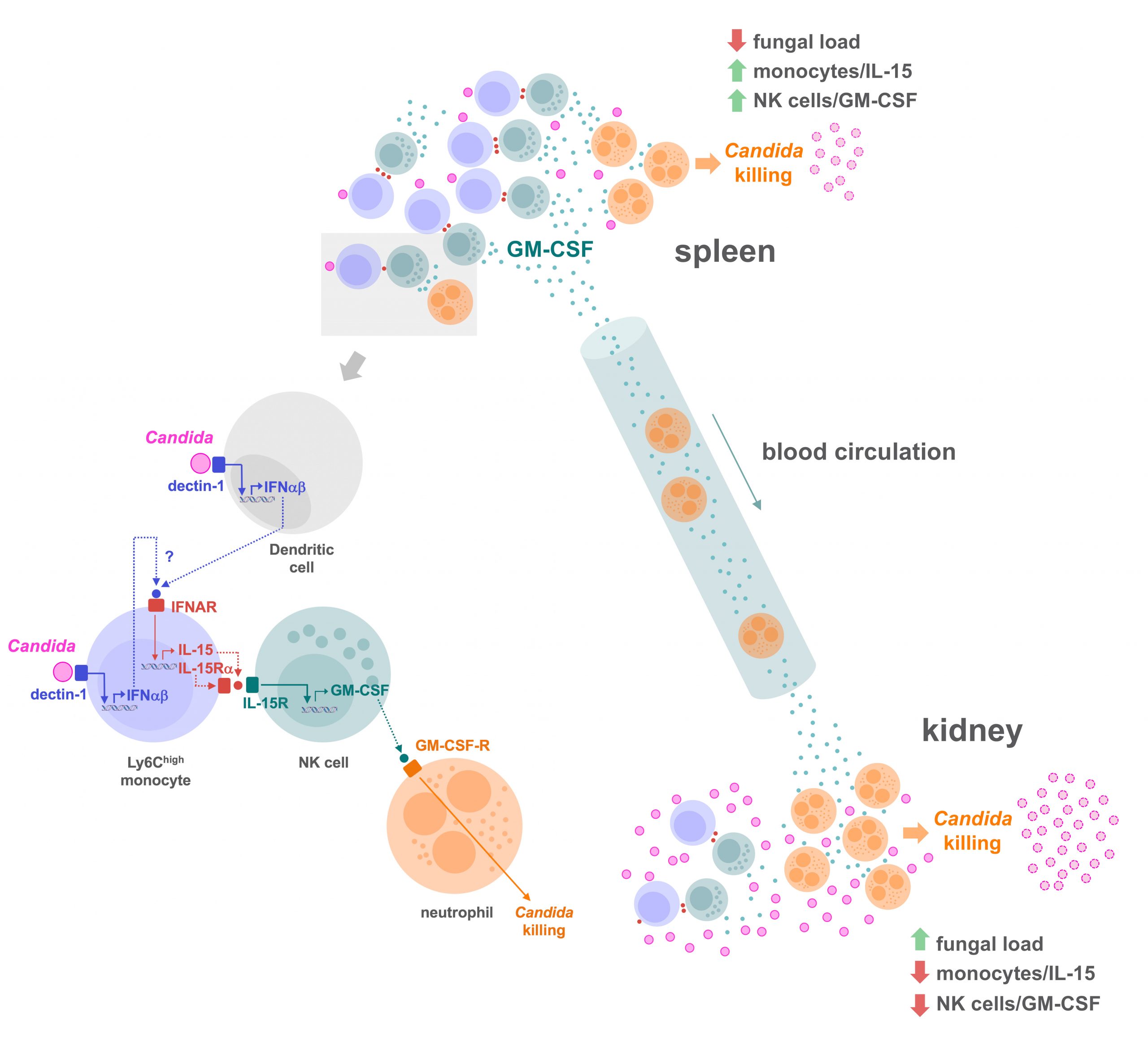
Using a murine model of systemic candidiasis to explore the role of inflammatory monocytes in NK cell-mediated neutrophil activation in defense against Candida albicans, we found that efficient anti-Candida immunity requires a collaborative response between the spleen and kidney, which relies on type I interferon-dependent IL-15 production by spleen inflammatory monocytes to drive efficient activation and GM-CSF release by spleen NK cells. This in turn is necessary to boost the Candida killing potential of kidney neutrophils (Domínguez-Andrés et al., Immunity 2017). These findings unveil a novel role for IL-15 as a critical mediator in defense against systemic candidiasis, and hold promise for the design of IL-15-based antifungal immunotherapies
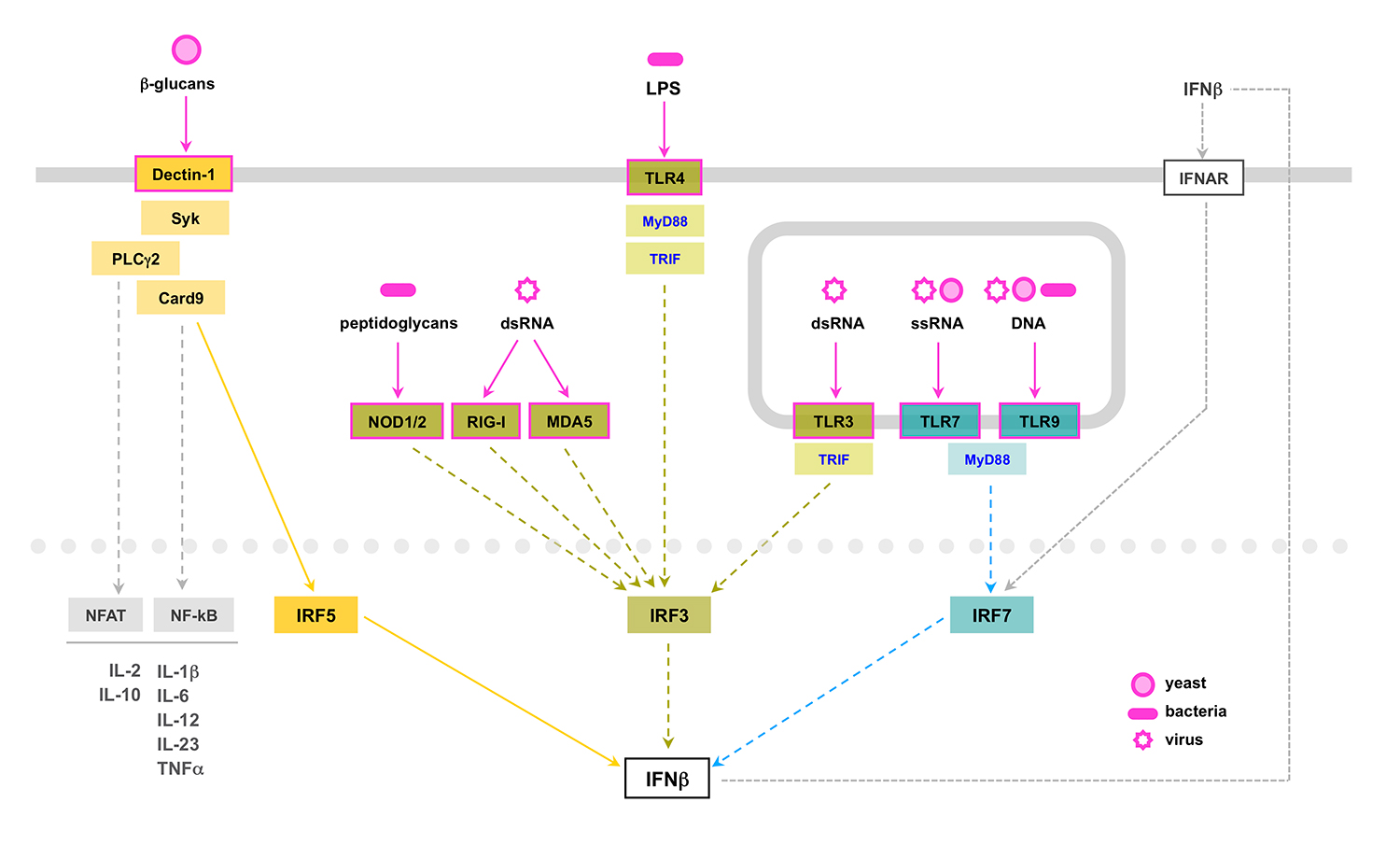
This project was a follow up of a previous study perfomed in our lab addressing the role in fungal infections of type I interferon, known for its role in antiviral and antibacterial immunity. After virus or bacteria recognition by cytosolic receptors or membrane-bound TLR receptors, type I interferon is produced following the activation of the transcription factors IRF3 or IRF7. Interestingly, our data revealed that type I interferon production by dendritic cells induced by C. albicans is triggered by a non-previously described signaling pathway initiated after engagement of the C-type lectin receptor Dectin-1, and dependent on the activation of the tyrosine kinase Syk and the transcription factor IRF5 (del Fresno et al., Immunity 2013).
These findings extend the physiological relevance of Dectin-1-mediated signaling in the context of the antifungal immunity since, in addition to driving the synthesis of cytokines essential for the induction of Th17 cell responses, it controls the activation of the NK cell/neutrophil axis through type I interferon-dependent IL-15 production.